TL;DR (quick summary)
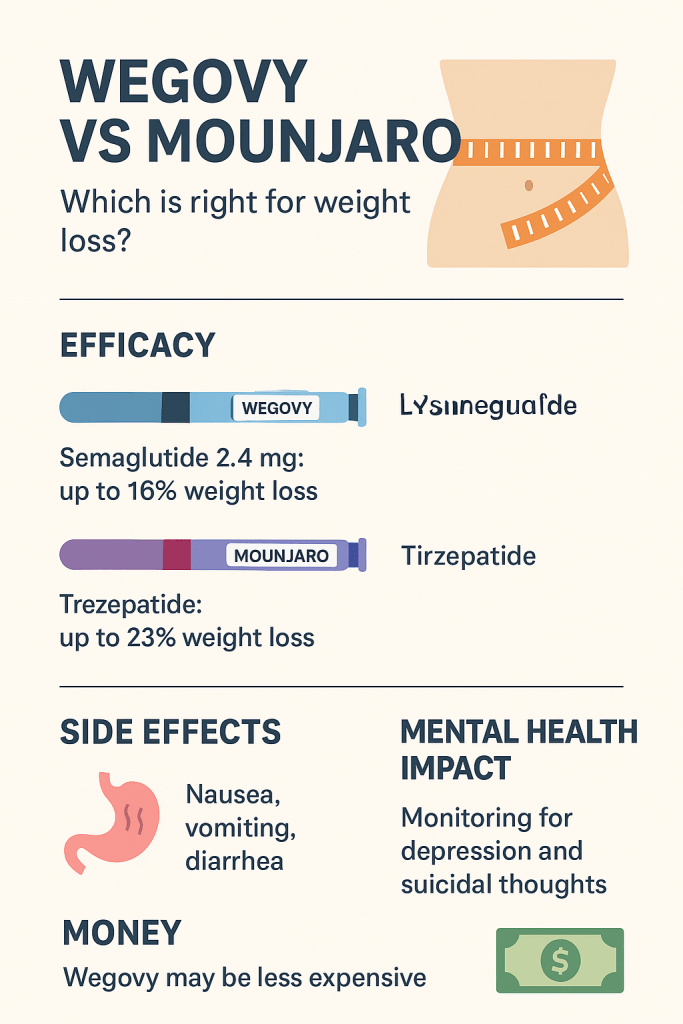
- Both Wegovy (semaglutide 2.4 mg) and tirzepatide (sold as Mounjaro for diabetes and Zepbound for chronic weight management) are weekly injectable medicines that powerfully reduce appetite and body weight.
- Tirzepatide (Zepbound / Mounjaro’s active ingredient) generally produces larger average weight loss in the major trials and in recent head-to-head data. Expect larger mean percent weight losses with tirzepatide at higher doses than with semaglutide 2.4 mg in the trials.
- Side effects are mostly gastrointestinal (nausea, vomiting, diarrhea, constipation), plus rare but important risks (pancreatitis, gallbladder disease, and warnings about thyroid C-cell tumors in rodents). Both drugs carry boxed warnings/important safety sections in their labels.
- Mental-health signals: semaglutide (Wegovy) labelling includes explicit language to monitor for new/worsening depression or suicidal thoughts; clinicians should monitor mood with any GLP-1/GIP therapy. Evidence on mood effects is evolving and mixed.
- Money: list prices for brand commercial fills are high (roughly $1,000–$1,400/month range), but manufacturer programs, pharmacy partnerships and insurer coverage vary widely. NovoNordisk (Wegovy) often provides patient programs and alternative cash pricing (e.g., NovoCare, retail discounts) that may make Wegovy financially competitive or even cheaper out of pocket in some situations; there is also recent reporting of large retail discounts (Costco). Separately, early real-world signals suggest Wegovy may have cardiovascular advantages that — if confirmed — could translate to long-term healthcare cost savings. Always check current pharmacy prices, insurer policy, and assistance programs for your situation.
1) How these drugs work
- Semaglutide (Wegovy) is a GLP-1 receptor agonist. GLP-1 is a gut hormone that reduces appetite, slows gastric emptying, and affects brain circuits that regulate hunger and reward — resulting in lower calorie intake and sustained weight reduction when combined with lifestyle changes.
- Tirzepatide (Mounjaro/Zepbound) is a dual GIP + GLP-1 receptor agonist (a single molecule that activates both receptors). The GIP component may add metabolic benefits — insulin sensitizing and additional appetite suppression — which is thought to be one reason tirzepatide produces larger average weight loss in trials.
2) How much weight can people expect to lose?
Semaglutide (Wegovy) — STEP trials
- In the pivotal STEP trials (semaglutide 2.4 mg once weekly plus lifestyle), participants lost large and clinically meaningful amounts of weight compared with placebo. STEP-1 showed sustained, clinically relevant mean weight loss (often ~15% or so in some reports across STEP programs depending on population and duration).
Tirzepatide (Zepbound / Mounjaro’s active ingredient) — SURMOUNT and SURMOUNT-1
- SURMOUNT-1 and related tirzepatide obesity trials reported mean weight losses that were greater numerically than semaglutide’s STEP results — ranges depend on dose and study length; high-dose tirzepatide produced average weight reductions in the ballpark of mid-teens to low-twenties percent at 72 weeks in the original trials (and higher in some dose groups). The landmark SURMOUNT trials showed 16–23% mean reductions depending on dose; longer-term patterns show many patients achieving ≥10%, ≥15% and even ≥20% weight loss.
Head-to-head data (most important recent study)
- A May 2025 head-to-head randomized trial published in NEJM directly compared tirzepatide vs semaglutide in adults with obesity and found greater weight reduction with tirzepatide over the study period (72 weeks), confirming what indirect comparisons and meta-analyses suggested. This is the strongest direct evidence we currently have that tirzepatide tends to produce larger average weight loss in comparable patients.
Bottom line on efficacy: tirzepatide (the dual agonist) appears to produce larger average weight loss than semaglutide 2.4 mg in trial settings, with semaglutide still producing substantial and clinically meaningful weight loss for many patients. Individual responses vary widely.
3) Side effects — what’s common and what’s rare but serious
Shared common side effects (both drugs)
- Nausea, vomiting, diarrhoea, constipation, abdominal pain, dyspepsia, and transient fatigue. These are dose-related and often improve over time as the body adapts.
Serious but rare adverse events
- Pancreatitis and gallbladder disease (including cholelithiasis) have been reported for both classes; monitor for severe abdominal pain or persistent vomiting.
- Thyroid C-cell tumors: Both labels include rodent findings of C-cell tumors and carry warnings; these findings have not been confirmed in humans but mean the drugs are contraindicated in people with a personal/family history of medullary thyroid carcinoma or MEN2 syndrome. The tirzepatide label explicitly warns about thyroid C-cell tumours.
- Hypoglycemia risk is higher when these drugs are used with insulin or sulfonylureas — important for people with diabetes.
Tolerability strategies
- Clinicians commonly use dose-escalation schedules to reduce GI side effects (start lower, increase every 4 weeks or per label), small meals, hydration, and anti-emetics if needed. Many patients experience the worst symptoms during the initial dose escalation and then improve.
4) Mental health impacts — what we know (and what we don’t)
- Wegovy’s FDA label includes a boxed (or prominent) warning/section to monitor for emergence or worsening of depression, suicidal thoughts or behavior and recommends avoiding Wegovy in patients with active suicidal ideation or prior suicide attempts. This language reflects both trial safety monitoring and signals seen with earlier weight-loss medications. Clinicians are advised to monitor mood.
- Tirzepatide/Zepbound/Mounjaro: psychiatric effects have been reported, but the formal labeling emphasizes the need for general clinical monitoring. The body of evidence on mood changes is still evolving — some users report improved mood with weight loss and improved metabolic health, others report new or worsened anxiety or mood symptoms (causation is not established).
Interpretation and practical advice
- Weight loss itself can improve quality of life and some mood symptoms for many people (better sleep, mobility, metabolic improvements). But rapid physiological changes, GI side effects, changes in appetite/hunger cues, or underlying psychiatric vulnerabilities can in some cases precipitate mood effects. Because of the label language for Wegovy (and prudence generally), clinicians should screen for depression history, monitor mood during therapy, and discuss any new or worsening symptoms promptly.
5) Regulatory differences and brand names
- Wegovy = semaglutide 2.4 mg — approved specifically for chronic weight management.
- Mounjaro = tirzepatide for type 2 diabetes (approved 2022). The weight-loss indication for tirzepatide is commercially available under the Zepbound brand (FDA approval for weight management in late 2023), while Mounjaro has historically been prescribed for diabetes. The active molecule is the same; brand and indication differ which affects insurance coverage in many markets. (Eli Lilly)
6) Financial comparison — cost, savings programs, and longer-term value
List / retail prices (examples & ballpark figures)
- Wegovy retail/list price has commonly been quoted around $1,300–$1,400 per 28-day pack, although actual cash prices and discounts vary. NovoCare and manufacturer offers sometimes provide a lower “cash” option (for uninsured) around $499/month in some programs (note: using such offers may process outside insurance).
- Mounjaro (tirzepatide) list price is commonly reported around $1,079.77 per fill (28-day), with Zepbound similar. Actual out-of-pocket depends on insurance, copays, and manufacturer coupons.
So — is Wegovy cheaper than Mounjaro?
- There’s no universal answer. By list price, Wegovy has often been similar or slightly higher than tirzepatide’s list price; however manufacturer programs and retailer deals can change the real out-of-pocket price quickly. For example, NovoNordisk’s NovoCare offers a $499/month cash path for some patients without insurance coverage, and recent news reported Costco making Wegovy/Ozempic available at substantially reduced cash prices — all of which can make Wegovy less expensive for certain patients at particular pharmacies or with particular programs. Meanwhile, Lilly has its own savings programs for tirzepatide that can also reduce patient cost. Always check current pharmacy prices and manufacturer offerings.
Potential long-term financial benefit of Wegovy (health-economics angle)
- A recent large real-world observational analysis reported that Wegovy was associated with a lower rate of major adverse cardiovascular events compared with tirzepatide in a cohort of patients with cardiovascular disease — suggesting a potential cardiovascular protection advantage with semaglutide in that dataset. If such a signal is confirmed in randomized trials and persists over time, reduced heart attacks/strokes could translate into large long-term healthcare cost savings (fewer hospitalizations, procedures, meds, disability). That is an important potential financial benefit of Wegovy over tirzepatide — but this evidence is observational and early, so treat it as promising but not definitive.
Insurance coverage reality
- Coverage policies differ greatly by insurer, country, and indication. In many places insurers require BMI thresholds and comorbidities to approve weight-loss drugs; some payers have tighter rules for weight-loss brand names than for drugs approved for diabetes. In the UK, for example, health authorities and NHS decisions have influenced how Mounjaro and Wegovy are reimbursed. Always check your insurer and speak to the prescribing clinician or pharmacist about prior authorization options and patient-assistance programs.
7) Practical considerations when choosing (clinical + lifestyle)
- If maximum weight loss is the primary goal: the trial data and head-to-head results suggest tirzepatide achieves higher average percent weight loss; consider Zepbound (weight-label tirzepatide) or discuss Mounjaro use off-label for weight under clinician guidance.
- If you have a history of depression or suicidal ideation: Wegovy’s label contains specific language to avoid use in active suicidal ideation and to monitor closely. That does not mean tirzepatide is safe in all such patients, but Wegovy’s labeling is particularly explicit — discuss psychiatric history with your prescriber.
- If you have diabetes and need glucose lowering: Mounjaro (for diabetes) might be preferred because it’s specifically approved for glycemic control; dosing strategies and concomitant diabetes medications need careful management to reduce hypoglycemia risk.
- If long-term cardiovascular risk reduction is a top priority: emerging signals favor semaglutide/Wegovy in some analyses for certain populations — but this area is evolving, and definitive randomized cardiovascular outcome comparisons are still being assessed. Discuss cardiovascular history and goals with your clinician.
8) Real-world and long-term considerations
- Durability of weight loss: longer-term follow-up of tirzepatide users suggests sustained, substantial weight loss over multiple years in many patients; semaglutide also shows durable effects while on therapy but weight regain is common after stopping medication. Both classes appear to require ongoing treatment (or some maintenance strategy) to preserve the effect.
- Access & supply: high demand has produced bottlenecks, pricing shifts, and a secondary market. Use only legitimate pharmacies and prescriptions. Pharmacies, retailers, and manufacturers are actively changing pricing/availability (e.g., retail discount programs).
9) Common patient questions (and short answers)
- “Which one will make me lose the most weight?” — On average, tirzepatide produces larger mean weight loss in trials and in a 2025 head-to-head study, but individual responses vary.
- “Are they safe for my mood?” — Both require monitoring. Wegovy’s label calls out monitoring for depression/suicidal thoughts; tirzepatide users should also be monitored for psychiatric changes. If you have current or past severe mental-health issues, talk to your prescriber first.
- “Which is cheaper?” — Depends on your insurance, pharmacy, and current manufacturer/retailer programs. Check coupons, NovoCare, manufacturer savings, GoodRx and big-retailer discounts (Costco has recently announced deep discounts on semaglutide pens). Always price check local pharmacies and talk to the clinic’s financial counsellor.
10) Practical blog-ready callouts / SEO-friendly bullets (copy/paste)
- Headline idea: “Wegovy vs Mounjaro: Efficacy, Side Effects, Mental-Health Signals and Which Might Save You Money”
- SEO bullets:
- “Wegovy (semaglutide) — how it works, average weight loss, and safety.”
- “Tirzepatide (Mounjaro/Zepbound) — dual GIP/GLP-1 action and why trial results show greater weight loss.”
- “Mental health and the weight-loss jabs: what the labels say and what to watch for.”
- “Money matters: list prices, patient programs, and real-world data that could affect long-term costs.”
11) Caveats, uncertainties and where research is headed
- The field is fast-moving: new comparative trials, cardiovascular outcome studies, and dose/formulation approvals appear regularly; pricing and access programs change quickly (retail discount programs, pharmacy partnerships). For the latest, check FDA labels, NEJM or large trial publications, and pharmacy/manufacturer announcements. I used the most relevant trial publications and FDA labels to build this summary.
12) Final thoughts — how to use this information
- Assess goals: weight loss amount desired, comorbidities (diabetes, heart disease, psychiatric history).
- Discuss safety: go over GI tolerability, rare risks (pancreatitis, gallbladder, thyroid concerns), and mental-health monitoring.
- Verify coverage & cost: check insurer prior authorization rules, pharmacy cash prices, and manufacturer programs (NovoCare, Lilly savings) to understand your likely out-of-pocket.
- Plan follow-up: regular clinician visits, labs as indicated, mood monitoring, and a plan for maintaining weight if/when medication changes.
References & selected sources
- Wilding JPH et al., NEJM — Once-Weekly Semaglutide (STEP trials). New England Journal of Medicine
- Jastreboff AM et al., NEJM — Tirzepatide for obesity (SURMOUNT). New England Journal of Medicine
- Direct head-to-head trial (May 11, 2025), NEJM — Tirzepatide compared with semaglutide (weight results). New England Journal of Medicine
- Wegovy (semaglutide) FDA label / prescribing information (monitoring for depression/suicidality; adverse reactions). FDA Access Data+1
- Mounjaro (tirzepatide) prescribing information (thyroid C-cell tumor warning, safety profile). FDA Access Data
- NovoCare / Wegovy pricing and patient support pages. NovoCare
- Retail/discount news: Costco announced reduced pricing for Wegovy/Ozempic (press coverage). People.com
- Recent real-world cardiovascular comparison reporting (Wegovy vs tirzepatide) — Reuters coverage of presented findings (Aug 2025). Reuters


Leave a comment